Robert McCarthy
Recently, I received an email from Research Gate that notified me as a co-author of a Jove 2012 publication, “Mouse islet of Langerhans isolation using a combination of purified collagenase and neutral protease,” that the article was read > 1500 times and it was viewed > 42,000 times. This article describes using a purified enzyme mixture specifically developed for rodent islet isolation.
The purified enzyme formulation used in the cited paper was developed by “reverse engineering” a “good lot” of Sigma Type XI collagenase used at Raghu Mirmira’s lab at the Indiana University School of Medicine. Enzyme activity analyses included the assay of collagenase’s collagen degrading activity and neutral protease activity using kinetic fluorescent microtiter plate assays.(1, 2) Based on these results, comparable enzyme formulations using purified Collagenase MA and BP Protease were prepared to match the enzyme activities found in the Type XI lot used as a reference material. The JOVE article emphasizes the advantages of using purified-defined collagenase-protease enzyme mixtures instead of Sigma Type XI collagenase for mouse islet isolation. However, notable observations made during the development of this enzyme mixture were excluded from this discussion. Moreover, there is no reference to VitaCyte’s launch of the CIzyme RI product for rodent islet isolation or later modifications to rodent islet isolation protocols.
Notable Observations From Development of Enzyme Mixtures for Mouse Islet Isolation
Several purified collagenase-neutral protease enzyme formulations were assessed for their effectiveness in recovering islets from C57BL/6 mice using the Gotoh procedure. These results showed differences between thermolysin and BP Protease when either were included in enzyme mixtures containing a fixed amount of collagenase. Islets isolated with thermolysin had a smaller size distribution, lower yields, and poorer morphology after short term culture when compared to those isolated with BP Protease. Islets isolated with BP Protease were of similar size and morphology to those islets isolated with Sigma Type XI. Others have shown the importance of protease selection for islet isolation.(3) Comparison of human islet morphologies using a split pancreas model showed a significantly higher percentage of islets with > 200 µm diameters for islets isolated using a fixed dose of collagenase and Clostridium histolyticum neutral protease than islets isolated with the same dose of collagenase and thermolysin.
Launch of CIzyme RI Product, Modification of the Islet Isolation Protocol
The report cited above was a research study where the purified collagenase and protease were mixed together at the time of use. VitaCyte launched the CIzyme RI product in 2012 that contained the enzyme formulation described above as a combined collagenase-protease mixture. Based on the results above, the recommended volume of the reconstituted product was 15 mL in an appropriate balanced salt solution with 2.5-3.0 ml injected into each mouse. This volume translates to one bottle of product recovering islets from 5-6 mice. Feedback from lead users who evaluated the CIzyme RI product stated that a similar Liberase product manufactured by Roche isolated islets from a larger number of mice. Comparison of the enzyme formulations of CIzyme RI with the Roche product indicated that these products were similar so the CIzyme RI could be diluted > 2 fold. An experienced islet isolator at a diabetes center on the east coast showed that the product could be diluted up to 30 mL for isolating mouse islets and to 40 mL for rat islet isolation without losing its ability to isolate islets. The mice and rat islet isolation protocols recommended in the CIzyme RI package insert are from this diabetes center. The majority of the results reported in VitaCyte’s white paper “What is an Acceptable Rodent Islet Yield?” was contributed by this same diabetes center, demonstrating the effectiveness of CIzyme RI to recover mouse or rat islets from different strains of mice or rats of different ages and raised on different diets.
Individual entries contain the following information:
- Rodent strain, supplier, age, weight, sex
- Average islet yield per pancreas
- Method used (Gotoh, Lacy, other)
- Product used including mass or activity per mL enzyme solution
- Digest time
- Purification procedure (Ficoll or Histopaque) with density g/mL
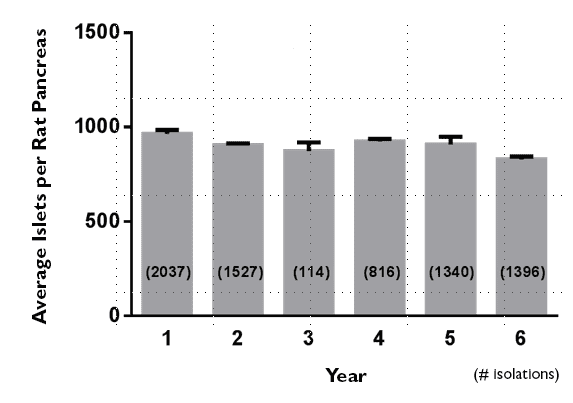
Several years ago, the diabetes center noted above summarized their results for isolating islets from 220-250 g Sprague Dawley rats over a 6 year period. They used 12 different lots of CIzyme RI product and found no adjustments were needed to the dose or timing of the pancreatic digestion procedure. These data illustrate the need for lot qualification was eliminated when a consistently manufactured collagenase-protease mixture was used for islet isolation.
References
1. McCarthy RC, Spurlin B, Wright MJ, Breite AG, Sturdevant LK, Dwulet CS, et al. Development and characterization of a collagen degradation assay to assess purified collagenase used in islet isolation. Transplantation Proceedings. 2008;40:339-42.
2. Breite AG, Dwulet FE, McCarthy RC. Tissue Dissociation Enzyme Neutral Protease Assessment. Transplantation proceedings. 2010;42:2052-4.
3. Balamurugan AN, Loganathan G, Bellin MD, Wilhelm JJ, Harmon J, Anazawa T, et al. A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products. Transplantation. 2012;93:693-702.
